Background: The incidence of SPM in transplant eligible MM patients (pts) receiving lenalidomide maintenance is ≥ 12% and is associated with inferior overall survival (OS). Recent evidence suggests that while clonal hematopoiesis does not increase the risk of SPM, specific TP53 mutant HPC may expand under lenalidomide treatment and give rise to therapy related myeloid neoplasms and acute lymphoblastic leukemia. Genetic screening may facilitate personalized treatment to minimize the risk of SPM. However, there are no longitudinal studies in purified HPC to inform on the evolution of specific gene mutations and its association with clinical outcomes.
Aim: Analyze the mutational landscape of HPC at diagnosis and throughout lenalidomide based regimens.
Methods: This study included 335 pts: 43 with high risk smoldering MM, 223 transplant eligible and 69 ineligible active MM respectively enrolled in the CESAR, GEM2012MENOS/GEM2014MAIN and CLARIDEX clinical trials. Of the 335 pts analyzed at diagnosis, 60 were further investigated after induction, 30 at day 100 after autologous transplant (ASCT) and 47 during maintenance. Two or more studies were available throughout treatment in 36 pts. Fluorescence activated cell sorting of CD34+ HPC was performed in a total of 500 bone marrow aspirates. DNA was analyzed with a panel of 55 genes recurrently mutated in myeloid neoplasms. Sequencing depth was >1000x in 95% of nucleotides. Criteria to filter out included synonymous, intronic, invalid-transcript, 5'UTR, panel error and single nucleotide polymorphisms. The criteria to filter in was a AMP/ACMG classification of uncertain significance, likely pathogenic or pathogenic. Only the latter two were included in downstream analysis.
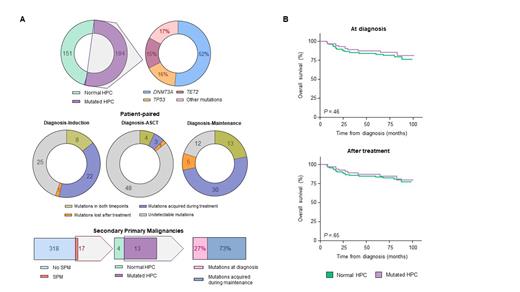
Results: HPC from 184 of the 335 (55%) pts were mutated. The frequency in high-risk smoldering MM, transplant eligible and ineligible active MM was 46%, 26% and 35%, respectively. The genes more frequently mutated were DNMT3A (n=94, 52%), TP53 (n=29, 16%) and TET2 (n=28, 15%). The median variant allele frequency was 4.9, 7.5 and 7.0, respectively.
In a pt-paired analysis between diagnosis and induction, 8 mutations were present in both time points, 25 became undetectable and 22 were acquired during treatment. The latter (eg, PPM1D and SF3B1) were observed in 14 of the 60 (23%) pts. In the 30 pts with paired samples at diagnosis and at day 100 after ASCT, 4 mutations were present in both time points, 48 became undetectable and only 3 were acquired during treatment ( ATRX, MPL and SAMDL9). Thus, the frequency of mutant HPC was higher at diagnosis vs ASCT (23% vs 5%, P <.001). In a pt-paired analysis between diagnosis and maintenance, 13 mutations were present in both time points, 12 became undetectable and 30 were acquired during treatment. The latter (eg, PPM1D and SF3B1) were observed in 24 of the 47 (51%) pts screened at both time points, and after a median of 5 years since diagnosis.
With a median follow-up of 6 years, 17 of the 335 (5%) pts developed SPM. Mutant HPC were detected in 13 of the 17 pts. Accordingly, presence of mutant HPC was associated with a 2-fold increased risk of SPM (OR: 2.3; P = .03). Of the 13 pts, 3 showed mutations at diagnosis and in 8 these emerged during maintenance. The only case developing a myeloid neoplasm had a TP53 mutation present during maintenance that was undetectable at diagnosis.
Pts with mutant vs non-mutated HPC at diagnosis showed similar OS (6y rates of 86% and 81%, respectively; P =.46). Similarly, there were no differences in OS of pts with emerging mutations vs those without mutant HPC after treatment (6y rates of 85% and 83%, respectively; P =.65). Specific mutations in DNMT3A, TP53 and TET2 and were not associated with inferior OS.
Conclusions: To our knowledge this is the first study analyzing the mutational landscape of purified HPC throughout lenalidomide based regimens in high risk smoldering MM as well as transplant eligible and ineligible active MM. Deep sequencing of CD34+ HPC uncovered that the frequency of mutations is similar between precursor and malignant disease, and that TP53 is amongst the top mutated genes.
There was considerable volatility with presence of both transient and emerging mutations throughout treatment. Importantly, the detection of mutant HPC was associated with increased risk of SPM without differences in survival. Thus, this study supports the longitudinal screening of HPC for the identification of patients at risk of developing SPM.
Disclosures
Puig:BMS: Consultancy, Honoraria, Other, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Other, Research Funding; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other, Research Funding; The Binding Site: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Other, Research Funding; Pfizer: Research Funding. Cedena:Janssen: Honoraria; BMS: Honoraria; Abbvie: Honoraria. Rocafiguera:Menarini: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Rosiñol:Takeda: Other: Honoraria for lectures; GlaxoSmithKline: Other: Honoraria for lectures; Sanofi: Other: Honoraria for lectures; Amgen: Other: Honoraria for lectures; Bristol Myers Squibb/Celgene: Other: Honoraria for lectures; Janssen: Other: Honoraria for lectures. Moraleda:Gilead-Kite: Honoraria; Novartis: Speakers Bureau; BMS/Celgene: Speakers Bureau; Roche: Speakers Bureau; Jazz Pharma: Other: Advisory board. Mateos:Takeda: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; University of Salamanca/Gerencia Regional de Salud de Castilla y León: Current Employment; Amgen: Honoraria; Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria; BMS-Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bladé:Celgene/Bristol Myers Squibb: Other: Honoraria for lectures; Sanofi: Other: Honoraria for lectures; Janssen: Other: Honoraria for lectures; Amgen: Other: Honoraria for lectures. San-Miguel:BMS: Other: Advisory Board; Janssen-Cilag: Other: Advisory Board; Karyopharm: Other: Advisory Board; Haemalogix: Other: Advisory Board; Amgen: Consultancy, Other: Advisory Board; Takeda: Other: Advisory Board; Novartis: Other; MSD: Other: Advisory Board; Abbvie: Consultancy, Other: Advisory Board; GSK: Other: Advisory Board; Roche: Other: Advisory Board; Celgene: Other: Advisory Board; Regeneron: Other: Advisory Board; Sanofi: Other: Advisory Board; SecuraBio: Other: Advisory Board. Paiva:Sanofi: Consultancy, Honoraria, Research Funding; GSK: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; EngMab: Research Funding; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Roche Glycart AG: Honoraria, Research Funding; Adaptive: Honoraria; Amgen: Honoraria; Gilead: Honoraria; Oncopeptides: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal